
TO SUBSCRIBE FOR NABTEB NOV/DEC GCE CHEMISTRY OBJ & PROSE ANSWERS VIA LINK ONLY
- JUST GO OUT AND BUY MTN CARDS OFN800 (200 + 200 + 200+200 = 800)
- GO TO YOUR MESSAGE, TYPE THE CARD PINS CORRECTLY AND SEND TO08065582389.
- DON’T CALL, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU CONFIRMING THAT YOU HAVE BEEN SUBSCRIBED.
- RELAX AND WAIT FOR YOUR ANSWERS 30MINUTES BEFORE EXAM STARTS OR AFTER EXAM STARTS.
- NB: DO NOT SEND USED CARD PINS OR YOUR NUMBER WILL BE BLACKLISTED.
NB: Only Share this Page with Trusted Students, We will be hiding this page immediately exam ends and a new page will be created for the upcoming exam. Kindly do well to bookmark the site and check back later.
==============================================
PAST NABTEB GCE CHEMISTRY ANSWERS
CHEMISTRY OBJ -OBJ
1BBBBCCABBC
11ADDCAACBDA
21BBADBBCAAD
31CCBCCBBADA
41ABCCCDBABA
Completed.
++++++++++++++++++++••++++++++++++++++••++++++++++++++++++++++++++++
CHEMISTRY- ESSAY
(1ai)
(a) Reduction; Copper
(b) Electrolytically; Aluminium
(1aii)
(i) It is ductile
(ii) It is malleable
(1aiii)
Metals are good conductors of electricity because they allow electric charge to flow freely through them
(1aiv)
(i) Copper : Brass
(ii) Aluminium : Alnico
(1bi)
Fluorine>Chlorine>Bromine
(1bii)
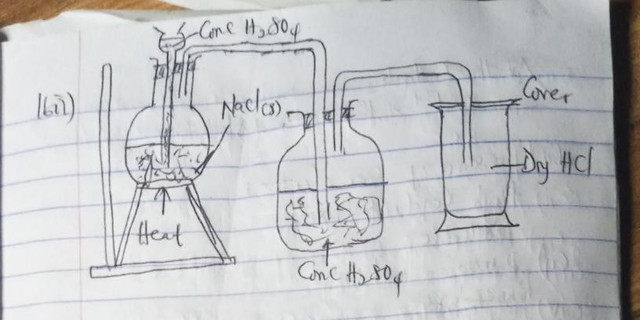
(1c)
(i) Water is Acidic
(ii) Water is a hard water
(iii) Water is an electrolyte
(1di)
Simple distillation
(1dii)
(i) Temperature
(ii) Pressure
++++++++++++++++++++••
(2ai)
(i) They form coloured ions of different charges
(ii) Some are very unreactive (silver and gold)
(iii) Many are used as catalysts
(2aii)
Zinc is regarded as non-transition metal because its compounds or ions (Zn²+) contain a full filled d-orbital or sub shell and are unstable.Moreover it have only one ion which is the (Zn²+). Hence zinc do not form colored aqueous ions because it do not have at least one vacant d-orbital in which it can receive an electron in a reaction.
(2aiii)
(i) Copper
(ii) Graphite
(iii) Brass
(iv) Silver
(2bi)
At the cathode;
Aluminium ions gains three electrons each at the cathode to become deposited as metallic aluminium:
Al³+ + 3e- => Al (reduction – gain electrons)
At the anode;
The oxygen ions donate two electrons each to form atomic oxygen, which then pair off to form gaseous molecules:
O²- => O + 2e-
(oxidation – lose electrons)
O + O => O²
(2bii)
(i)It is soft
(ii)It is malleable
(ii)It is ductile
(2ci)
=Experiment to describe Brownian movement=
Add some powered sulphur to water and stir. Then filter the suspended sulphur. Some of the sulphur particles are small enough to pass through the pores of the filter paper and hence, stay in the filtrate as a colloidal suspension. Put a drop of the filtrate on a slide and and examine it under a power microscope.
Result: The sulphur particles are observed to move about rapidly all the time in a random and zig zag manner in the medium.
Conclusion: The brownian movement of the sulphur particles reflects the movement of the water molecules
(2cii)
(i) Alnico
(ii) Duralumin
(2ciii)
(i) SO²
(ii) SO³
++++++++++++++++++++••
(3ai)
(i)Neutralization of a base by an acid
(ii)Direct combination of elements
(3aii)
(a) Normal salts: Sodium salt(NaCl)
(b) Acid salt: Sodium hydrogen sulfated(NaHSO⁴)
(c) Basic salt: Sodium acetate (NaOOCCH³)
(3aiii)
Oxidation reaction
(3bi)
(i)The particles of a substance constantly move in random motion, and during this random motion, they collide with each other and with the walls of the container.
(ii)All collisions between gas molecules are perfectly elastic; all kinetic energy is conserved.
(iii)During two successive collisions, the average distance moved by the particle (between two collisions) is called ‘mean free path’.
(3bii)
(a) Gas molecules are in a state if random motion colliding with one another and the wall of the vessel
(b) Volume occupied by gas molecules are negligible compared to the volume of the container
(c) The average kinetic energy of gas molecules is directly proportional to the absolute temperature
(d) Hydrogen gas is evolved
(3biii)
Ca>Al>Zn>Fe
(3ci)
Fine chemicals are chemicals produced in small quantities for specific purposes and to a very high degree of purity WHILE Heavy chemicals are chemicals used extensively in industries and are produced in very large quantities.
(3cii)
(i) Chlorine: It is used to kill bacteria and other harmful substances in water
(ii) Chloramine: It is used to disinfect water
(3ciii)
Release of freons from aerosol
(3civ)
Use filters for chimneys
(3di)
By passing H2S into FeCl2
2FeCl2 + H2S => 2FeCl2 + 2HCl + S
Iron was reduced from +3 to +2 by S
(3dii)
CH3CH2OH + Cl2 => CH3CHO + HCl ( removal of hydrogen ie oxidation)
++++++++++++++++++++••
(5a)
Le chatelier’s principle states that if an external constraint such as changes in temperature, changes in pressure or changes in concentration is imposed in a chemical system in equilibrium, the equilibrium will shift so as to annul or neutralise the constraints
(5b)
(i) No effect
(ii) The equilibrium will shift to the right
(iii) No effect
(5ci)
(a)Hydrogen gas
(b) (i) Cathode reaction
2H^+ + 2e- => H2(g)
(ii) Cathode reaction
4OH => 2H2O + 2O2(g) + 4e-
(5cii)
IF = 96500c
2F = x
Q = 193000c
m = 64*193,000/2*96500
= 64g
(5di)
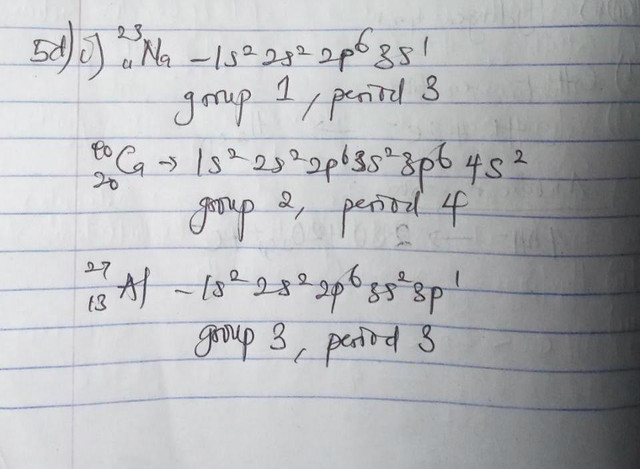
++++++++++++++++++++••
+++++++++++++++++++++++++++
COMPLETED!!
Please I’m having difficulty answers for the Adv. Financial Accounting of today.
Please how do I access the NABTEB Chemistry for tomorrow, 30th November?
Good evening. Please do you have answers for the following NABTEB 2023 N/D GCE exam:
1. Block laying/Bricklaying and Concreting;
2. Catering Craft Practice;
3. Plumbing & Pipe Fitting;
4. Building Science;
5. Mechanical Engineering Science.
Thanks.